Connecting (organo)silica sol-gel synthesis and electrospinning: pushing toward molecular scale-driven property tuning
Linking the chemical structure to the properties of silica nanofibers
(Organo)silica nanofibrous membranes are porous, flexible and resistant to high temperatures and harsh chemical conditions. This unique combination of properties makes them highly suitable for advanced applications, but today their full potential cannot be exploited due to a lack of fundamental knowledge and a straightforward way to produce them. To overcome this, advanced kinetic modeling techniques and experimental tools are combined allowing to bridge the molecular and the materials scale.
This research aims to enable the production of materials that meet the following four criteria: they are (i) flexible, (ii) resistant to harsh chemical conditions, (iii) resistant to high temperatures and (iv) have a porous structure. Such materials would be of use in various stringent problems such as water filtration in developing countries, separation of hot or acid exhaust gases of factories, the separation of dispersed liquids and as catalyst carrier for the conversion of CO2 to fuels. Silica is a material that meets two of the four listed criteria. Better known in its common shapes glass, quartz and white sand, silica is known for its very high chemical and thermal resistance. However, in these common shapes, it is neither flexible nor porous. An interesting pathway is to look at silica materials in a different shape, so that more than two of the criteria can be fulfilled. One option is to make nanofibrous membranes of silica. Nanofibrous membranes are inherently highly porous and flexible. They consist of randomly deposited (continuous) nanofibers, which are fibers with a diameter < 1000 nm. Nanofibrous membranes made from silica have already been produced and have shown to be indeed flexible, resistant to harsh chemical conditions and high temperatures and porous. However, their usability is limited because their production process – called ‘electrospinning’ – is not straightforward and not well understood.
In the electrospinning process, a spinning solution in a syringe is subjected to a high voltage (10-30 kV) which creates repulsive charges within the spinning solution. As a result, a very thin fiber is drawn from the drop coming out of the tip of the needle. This fiber gets stretched out on its spiraling way to the grounded collector plate. The random deposition of the nanofiber creates a nanofibrous membrane.
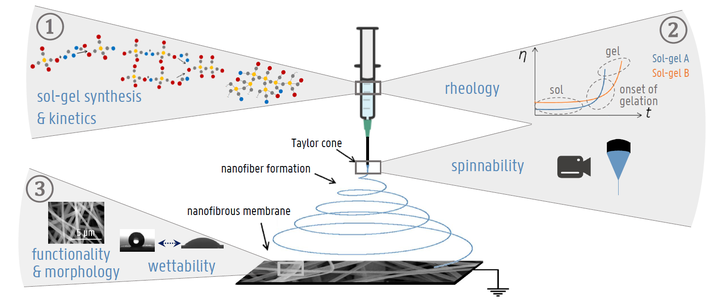
There are three interlinked parts in this research:
- Fundamental insights in the silica spinning solution. The preparation of the silica spinning solution which is subsequently used for electrospinning is done by a sol-gel process. Combining kinetic modeling techniques and experiments leads to insights in the chemical reactions of this process.
- Electrospinning of the silica spinning solutions made in step 1. The goal is to understand which silica spinning solutions give rise to uniform nanofibers, and how this relates back to the molecular structure of the spinning solution. For this, the flow behavior of the silica spinning solution is investigated.
- Control of the resulting nanofibrous membranes. The morphology of the nanofibrous membranes made in step 2 is investigated to gain a better understanding of the process in order to tune the nanofiber thickness. The wettability and adhesion properties of the nanofibrous membranes are studied, to find an answer to how the molecular composition affects the resulting membrane properties.
Hence, this research focuses on understanding how the molecular scale gives rise to nanofibrous membranes and how it influences the properties of those membranes, bridging the molecular scale (step 1) and the material scale (step 3).
Acknowledgements
This research is funded by the FWO – Research Foundation Flanders by an FO PhD grant (1169122N), by the Belgian-American Educational Foundation and by Fulbright. Ir. Sofie Verschraegen conducts the research at the Centre for Textile Science and Engineering (Ghent University) in collaboration with the Laboratory for Chemical Technology (Ghent University), the Sol-gel Centre for Research on Inorganic Powders and Thin Films Synthesis (Ghent University) and the Dauskardt group (Stanford University).
Contact
Prof. dr. ir. Karen De Clerck (Karen.DeClerck@UGent.be)