alpha-Galactosylceramide analogues
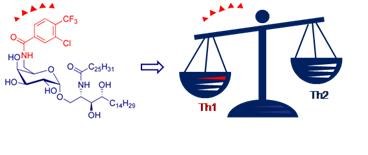
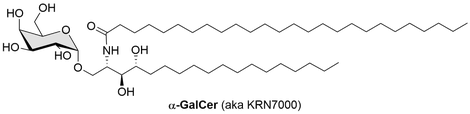
The processing and presentation of lipid antigens by antigen presenting cells (APC) is important for defense against infection, tumor immunosurveillance and autoimmunity. During the past years the use of glycolipids as immunostimulating agents has become increasingly important. When presented by the major histocompatibility complex (MHC) class I-like molecule CD1d, certain glycolipids are recognized by the semi-invariant T cell receptors (TCR) of natural killer T (NKT) cells. The prototypical antigen for NKT cells is a-galactosylceramide (a-GalCer), which was obtained by structural optimization of agelasphins (a-linked glycosphingolipids isolated from a marine sponge). Upon recognition of the CD1d-a-GalCer bimolecular complex by their TCR, NKT cells are activated, resulting in the rapid release of T helper 1 (Th1) and T helper 2 (Th2) cytokines. As both Th1 and Th2 cytokines influence the outcome of different immune responses, disruption of the carefully controlled Th1/Th2 balance can lead to disease induction and progression.
While certain autoimmune diseases are characteristic of hyporesponsiveness to Th2 and overactivation of pathogenic Th1 cells, the opposite is true for many types of cancer that have a predominant Th2 response. Hence, a-GalCer analogues that induce a biased Th1/Th2 response are highly awaited.
Most of the known a-GalCer analogues able to induce polarized cytokine responses are characterized by modifications of the phytosphingosine or fatty acyl chains, expected to alter the affinity for CD1d.
We identified a series of 6’-derivatized a-GalCer analogues with an intact phytoceramide moiety, which are capable of skewing the cytokine release profile to Th1 and possess a comparable ability to induce INF-γ secretion as a-GalCer. In contrast to modifications of other Gal OH-groups, these analogues clearly retain antigenic activity.