PROTAC TOOLKIT
Traditional approaches to treat diseases typically utilize a chemical agent to modulate the activity of (a) selected protein(s), e.g. enzyme inhibitors. A different approach utilizing Proteolysis Targeting Chimeras (PROTACs) was developed by the group of Craig Crews at Yale university.[1-2] This involves the active and specific lowering of the cellular abundance of proteins resulting in a reduction of their harmful activities. To achieve this, it employs the cellular Ubiquitin-Proteasome-System, which has been shown to degrade proteins that have been (poly)ubiquitinated.[3]
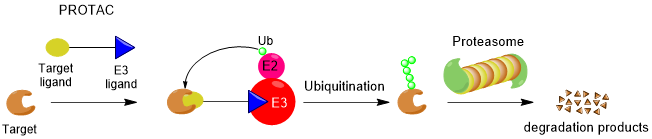
Figure 1: Mechanism of PROTAC mediated protein degradation.
Selective forced Ubiquitination of the offending proteins is achieved by the application of PROTACs: heterodimeric ligands built up from an E3-ligase ligand, a ligand for the target protein and a linker moiety connecting the two ligands. A PROTAC will thus induce the formation of a ternary complex with an E3-ligase and the target protein, thereby inducing (by proximity) (poly)ubiquitination of the target protein. This polyubiquitin tag acts as a signal for degradation of the protein by the proteasome after which the PROTAC is released again in the cytosol. Here it can act in successive cycles of trimerization and ubiquitination. PROTACs can thus be considered catalytic in nature.
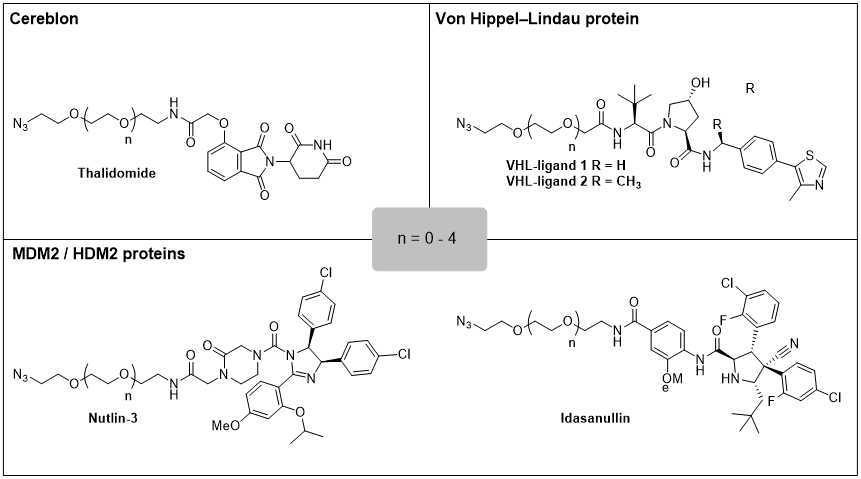
Table 1: PROTAC toolkit reagents
In our research, we utilize PROTACs as tools in the fields of oncology and immunology. To enable expedient PROTAC synthesis we have designed and synthesized a toolkit of general purpose PROTACs reagents that combine the E3-ligase ligand and the linker moiety in one molecule. The terminal end of the linker moiety comes pre-equipped with an azide moiety. With these reagents in hand, it only requires a ligand for the target protein equipped with a terminal alkyne and a simple CuAAC reaction[4] to prepare a sizable pool or PROTACs in a minimal amount of time. Reagents with ligands for several E3-ligases, including Cereblon, VHL and MDM2, have been prepared. [5-9]
Figure 2: PROTAC synthesis based on the toolkit reagent and a target ligand equipped with a terminal alkyne.
References:
[1] Sakamoto K.M.; Kim K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box
complex for ubiquitination and degradation Proc. Natl. Acad. Sci. U.S.A. 2001, 98(15), 8554-8559
[2] Schapira M.; Calabrese, M.F.; Bullock, A.N.; Crews C.M. Targeted protein degradation: expanding the toolbox Nat. Rev. Drug Discov. 2019, 18(12), 949-963.
[3] https://www.nobelprize.org/prizes/chemistry/2004/popular-information/ (accessed April 2020)
[4] Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective Ligation of Azides and
Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41(14), 2596-2599.
[5] Lohbeck, J. and Miller A.K. Practical synthesis of a phthalimide-based Cereblon ligand to enable PROTAC development.
Bioorg. Med. Chem. Lett. 2016, 26(21), 5260-5262.
[6] Galdeano, C.; Gadd, M.S.; Soares, P.; Scaffidi, S.; Van Molle, I.; Birced, I.; Hewitt, S.; Dias, D.M.; Ciulli, A. Structure-guided design and optimization of small
molecules targeting the protein-protein interaction between the von Hippel-Lindau (VHL) E3 ubiquitin ligase and the hypoxia inducible factor (HIF) alpha
subunit with in vitro nanomolar affinities. J. Med. Chem. 2014, 57(20), 8657-63.
[7] Raina, K.; Lu, J.; Qian, Y.; Altieri, M.; Gordon, D.; Rossi, A.M.; Wang, J.; Chen, X.; Dong, H.; Siu, K.; Winkler, J.D.; Crew, A.P.; Crews, C.M.; Coleman, K.G. PROTAC-induced
BET protein degradation as a therapy for castration-resistant prostate cancer. Proc. Natl. Acad. Sci. U.S.A. 2016, 113(26), 7124-7129.
[8] Schneekloth, A.R.; Pucheault, M.; Tae, H.S.; Crews, C.M. Targeted intracellular protein degradation induced by a small molecule: En route to chemical
proteomics. Bioorg. Med. Chem. Lett. 2008, 18(22):5904-5908
[9] Rimmler, G.; Alker, A.; Bosco, M.; Diodone, R.; Fishlock, D.; Hildbrand, S.; Kuhn, B.; Moessner, C.; Peters, C.; Rege, P.D.; Schantz, M. Practical Synthesis of MDM2
Antagonist RG7388. Part 2: Development of the Cu(I) Catalyzed [3 + 2] Asymmetric Cycloaddition Process for the Manufacture of Idasanutlin.
Org. Process Res. Dev. 2016, 20(12), 2057-2066.