Translational IVF research
Within this line of research, the 'Reproductive Medicine Research Group' focuses on the development of novel diagnostic tools and alternative treatment options for patients experiencing failed ART. Research is always conducted under the supervision of prof. dr. Björn Heindryckx en dr. Annekatrien Boel (post-doc).
Research projects
Failed fertilization with intracytoplasmic sperm injection (ICSI)
Injecting the sperm into the egg leads, in the majority of cases, to fertilization and the development of an embryo. Still, there is a population of patients who have repeated failed fertilization (FF) after this intracytoplasmic sperm injection (ICSI). Furthermore, it is also possible that the embryo development during the first division stages is repeatedly compromised (embryo developmental arrest – EDA).
To assess FF, we have developed a diagnostic test in mice, by which we assess the oocyte-activating capacity of human sperm cells, after ICSI in mouse oocytes (mouse oocyte activation test – MOAT). Furthermore, we can also more specifically evaluate the calcium pattern, induced by sperm cells, after ICSI in mouse (mouse oocyte calcium analysis – MOCA), or human (human oocyte calcium analysis – HOCA) oocytes.
To treat FF, the Department for Reproductive Medicine has obtained worldwide expertise, by evoking artificial calcium rises (assisted oocyte activation – AOA). With this AOA technique, most couples experiencing failed fertilization after ICSI significantly increase their chances to obtain a genetically related child, however for embryo developmental arrest, no treatment options other than oocyte donation are available.
In this research line, we investigate the molecular mechanisms underlying FF and EDA and aim to develop improved diagnostic and treatment methods.
- researcher: Arantxa Cardona Barberán, Annekatrien Boel, Yongqian Li
- funding: Funding for doctoral research of AAP (assisting academic staff), Ghent University, Research Foundation (FWO) and China Scholarship Council (CSC)
Study of early embryo developmental processes
Genes that contribute to the early development of mouse and human embryos have recently become easier to study by targeted gene disruption via the genome-modifying technique CRISPR/Cas. Also, the disruption of genes that play a crucial role in later developmental stages, such as embryo differentiation, implantation and stem cell derivation, is being investigated.
- researcher: Panagiotis Stamatiadis, Gwenny Cosemans
- funding: Research Foundation (FWO)
Mitochondrial disorders
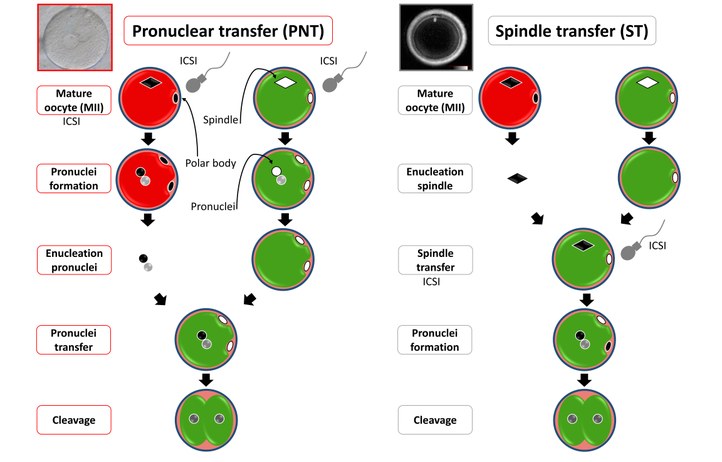
Mitochondrial disorders, caused by mitochondrial DNA mutations, which are always inherited from mother to child, can be prevented by either pre-implantation genetic testing (PGT) or nuclear transfer. This nuclear transfer procedure involves the transfer of the nucleus from the mother's egg to a healthy donor egg, containing healthy mitochondrial DNA. In the general press, this technique is also called the "three-parent baby".
Different nuclear transfer technologies are currently being evaluated both in mice and humans, amongst which spindle transfer and nuclear transfer (see figure) are the most well-known.
In addition to investigating nuclear transfer in the context of overcoming mitochondrial disorders, we also want to generate proof that the technique is valuable to overcome certain forms of female infertility, such as failed fertilization after ICSI-AOA, embryo developmental arrest and a limited ovarian reserve. For these women, oocyte donation is currently the only treatment option.
By using both mouse models as well as human cases, we aim to indicate which forms of female infertility could potentially benefit from this novel nuclear transfer technique.
- researcher: Maoxing Tang, Antonia Christodoulaki, Noemi Castelluccio, Yongqian Li
- funding: Research Foundation (FWO) , Special Research Fund (BOF-GOA), funding for doctoral research of AAP (assisting academic staff), Ghent University and China Scholarship Council (CSC)
Germline mutation correction
With the genome-editing technique CRISPR/Cas, it is possible to correct genetic mutations during fertilization. Our research is currently focused on the correction of mutations that lead to infertility, to prevent that babies, born after certain fertility treatments, will also suffer from infertility.
- researcher: Bieke Bekaert, Annekatrien Boel
- funding: Research Foundation (FWO)
Collaborations
- Center for Medical Genetics Ghent (CMGG), Ghent University (Prof. Paul Coucke, Prof. Björn Menten)
- Faculty of Pharmaceutical Sciences, Ghent University (Prof. Filip Van Nieuwenburgh, Prof. Dieter Deforce)
- Physiology, Ghent University (Prof. Luc Leybaert)
- Leiden University Medical Center (Prof. Susana Chuva de Sousa Lopes)
- Pharmacology group, Oxford University (Prof. John Parrington)
- Stem Cell Institute, KU Leuven (Dr. Frederic Lluis)