Publications before 2013
2012
41. Aromatic capping surprisingly stabilizes furan moieties in peptides against acidic degradation
Kurt Hoogewijs (UGent) , Annelies Deceuninck (UGent) and Annemieke Madder (UGent)
We herein describe the synthesis of furan containing peptides for further post-synthetic derivatisation in solution through our recently developed furan-oxidation-labeling technology. Previously, it was reported by others that during acidic cleavage of furan-modified peptides, furan moieties can suffer from degradation. We demonstrate here that this degradation is position dependent and can be fully suppressed through introduction of proximate aromatic residues. Versatile introduction of 2-furylalanine at internal, C-terminal as well as the sensitive N-terminal positions has now been proven possible.
(2012) ORGANIC & BIOMOLECULAR CHEMISTRY. 10(20). p.3999-4002
40. Fluorescent castasterone reveals BRI1 signaling from the plasma membrane
Niloufer Irani (UGent) , Simone Di Rubbo (UGent) , Evelien Mylle (UGent) , Jos Van den Begin (UGent) , Joanna Pizon (UGent) , Jaroslava Hniliková, Miroslav Šiša, Dieter Buyst (UGent) , Josep Vilarrasa-Blasi, Anna Maria Szatmari (UGent) , et al.
Receptor-mediated endocytosis is an integral part of signal transduction as it mediates signal attenuation and provides spatial and temporal dimensions to signaling events. One of the best-studied leucine-rich repeat receptor–like kinases in plants, BRASSINOSTEROID INSENSITIVE 1 (BRI1), perceives its ligand, the brassinosteroid (BR) hormone, at the cell surface and is constitutively endocytosed. However, the importance of endocytosis for BR signaling remains unclear. Here we developed a bioactive, fluorescent BR analog, Alexa Fluor 647–castasterone (AFCS), and visualized the endocytosis of BRI1–AFCS complexes in living Arabidopsis thaliana cells. Impairment of endocytosis dependent on clathrin and the guanine nucleotide exchange factor for ARF GTPases (ARF-GEF) GNOM enhanced BR signaling by retaining active BRI1-ligand complexes at the plasma membrane. Increasing the trans-Golgi network/early endosome pool of BRI1–BR complexes did not affect BR signaling. Our findings provide what is to our knowledge the first visualization of receptor-ligand complexes in plants and reveal clathrin- and ARF-GEF–dependent endocytic regulation of BR signaling from the plasma membrane.
(2012) NATURE CHEMICAL BIOLOGY. 8(6). p.583-589
39. GOLVEN secretory peptides regulate auxin carrier turnover during plant gravitropic responses
Ryan Whitford (UGent) , Ana Fernandez Salina (UGent) , Ricardo Tejos Ulloa (UGent) , Amparo Cuéllar Pérez (UGent) , Jürgen Kleine-Vehn (UGent) , Steffen Vanneste (UGent) , Andrzej Drozdzecki (UGent) , Johannes Leitner, Lindy Abas, Maarten Aerts (UGent) , et al.
Growth and development are coordinated by an array of intercellular communications. Known plant signaling molecules include phytohormones and hormone peptides. Although both classes can be implicated in the same developmental processes, little is known about the interplay between phytohormone action and peptide signaling within the cellular microenvironment. We show that genes coding for small secretory peptides, designated GOLVEN (GLV), modulate the distribution of the phytohormone auxin. The deregulation of the GLV function impairs the formation of auxin gradients and alters the reorientation of shoots and roots after a gravity stimulus. Specifically, the GLV signal modulates the trafficking dynamics of the auxin efflux carrier PIN-FORMED2 involved in root tropic responses and meristem organization. Our work links the local action of secretory peptides with phytohormone transport.
(2012) DEVELOPMENTAL CELL. 22(3). p.678-685
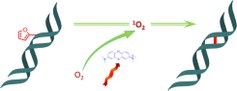
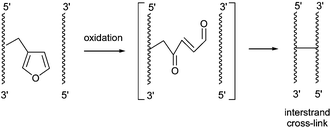
38. Sequence specific DNA cross-linking triggered by visible light
Marieke Op de Beeck and Annemieke Madder (UGent)
A new biocompatible strategy for photoinduced DNA interstrand cross-linking is presented. Methylene blue induced 1O2 formation triggers furan oxidation; the resulting aldehyde then rapidly reacts with complementary A or C with formation of stable adducts. Easily accessible furan modified nucleosides, a commercially available photosensitizer, and visible light irradiation constitute the necessary tools to achieve selective duplex interstrand cross-linking.
(2012) JOURNAL OF THE AMERICAN CHEMICAL SOCIETY. 134(26). p.10737-10740
37. Inactivated virus vaccines from chemistry to prophylaxis: merits, risks and challenges
Iris Delrue (UGent) , Dieter Verzele (UGent) , Annemieke Madder (UGent) and Hans Nauwynck (UGent)
The aim of this review is to make researchers aware of the benefits of an efficient quality control system for prediction of a developed vaccine's efficacy. Two major goals should be addressed when inactivating a virus for vaccine purposes: first, the infectious virus should be inactivated completely in order to be safe, and second, the viral epitopes important for the induction of protective immunity should be conserved after inactivation in order to have an antigen of high quality. Therefore, some problems associated with the virus inactivation process, such as virus aggregate formation, protein crosslinking, protein denaturation and degradation should be addressed before testing an inactivated vaccine in vivo.
(2012) EXPERT REVIEW OF VACCINES. 11(6). p.695-719
36. Fast RNA conjugations on solid phase by strain-promoted cycloadditions
Ishwar Singh, Colin Freeman, Annemieke Madder (UGent) , Joseph S Vyle and Frances Heaney
Strain promoted cycloaddition is presented as a tool for RNA conjugation on the solid phase; RNA-cyclooctyne conjugates are prepared by cycloaddition to both azide (strain-promoted azide-alkyne cycloaddition, SPAAC) and nitrile oxide dipoles (strain-promoted nitrile oxide-alkyne cycloaddition, SPNOAC). The conjugation is compatible with 2'-OMe blocks and with 2'-O-TBDMS protection on the ribose moieties of the sugar. Nitrile oxide dipoles are found to be more reactive click partners than azides. The conjugation proceeds within 10 min in aqueous solvents, at room temperature without any metal catalyst and tolerates dipoles of varying steric bulk and electronic demands, including pyrenyl, coumarin and dabcyl derivatives.
(2012) ORGANIC & BIOMOLECULAR CHEMISTRY. 10(33). p.6633-6639
2011
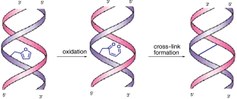
35. Furan-oxidation-triggered inducible DNA cross-linking: acyclic versus cyclic furan-containing building blocks-on the benefit of restoring the cyclic sugar backbone
Kristof Stevens (UGent) , Diederica Claeys (UGent) , Saron Catak (UGent) , Sara Figaroli, Michal Hocek, Jan M Tromp, Stefan Schurch, Veronique Van Speybroeck (UGent) and Annemieke Madder (UGent)
Oligodeoxynucleotides incorporating a reactive functionality can cause irreversible cross-linking to the target sequence and have been widely studied for their potential in inhibition of gene expression or development of diagnostic probes for gene analysis. Reactive oligonucleotides further show potential in a supramolecular context for the construction of nanometer-sized DNA-based objects. Inspired by the cytochrome P450 catalyzed transformation of furan into a reactive enal species, we recently introduced a furan-oxidation-based methodology for cross-linking of nucleic acids. Previous experiments using a simple acyclic building block equipped with a furan moiety for incorporation into oligodeoxynucleotides have shown that cross-linking occurs in a very fast and efficient way and that substantial amounts of stable, site-selectively cross-linked species can be isolated. Given the destabilization of duplexes observed upon introduction of the initially designed furan-modified building block into DNA duplexes, we explore here the potential benefits of two new building blocks featuring an extended aromatic system and a restored cyclic backbone. Thorough experimental analysis of cross-linking reactions in a series of contexts, combined with theoretical calculations, permit structural characterization of the formed species and allow assessment of the origin of the enhanced cross-link selectivity. Our experiments clearly show that the modular nature of the furan-modified building blocks used in the current cross-linking strategy allow for fine tuning of both yield and selectivity of the interstrand cross-linking reaction.
(2011) CHEMISTRY-A EUROPEAN JOURNAL. 17(25). p.6940-6953
34. Unprecedented C-selective interstrand cross-linking through in situ oxidation of Furan-Modified Oligodeoxynucleotides
Marieke Op de Beeck and Annemieke Madder (UGent)
Chemical reagents that form interstrand cross-links have been used for a long time in cancer therapy. They covalently link two strands of DNA, thereby blocking transcription. Cross-link repair enzymes, however, can restore the transcription processes, causing resistance to certain anti-cancer drugs. The mechanism of these cross-link repair processes has not yet been fully revealed. One of the obstacles in this study is the lack of sufficient amounts of well-defined, stable, cross-linked duplexes to study the pathways of cross-link repair enzymes. Our group has developed a cross-link strategy where a furan moiety is incorporated into oligodeoxynucleotides (ODNs). These furan-modified nucleic acids can form interstrand cross-links upon selective furan oxidation with N-bromosuccinimide. We here report on the incorporation of the furan moiety at the 2'-position of a uridine through an amido or ureido linker. The resulting modified ODNs display an unprecedented selectivity for cross-linking toward a cytidine opposite the modified residue, forming one specific cross-linked duplex, which could be isolated in good yield. Furthermore, the structure of the formed cross-linked duplexes could be unambiguously characterized.
(2011) JOURNAL OF THE AMERICAN CHEMICAL SOCIETY. 133(4). p.796-807
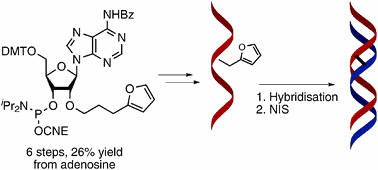
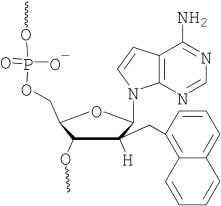
33. Synthesis and incorporation of a furan-modified adenosine building block for DNA interstrand crosslinking
Anup M Jawalekar, Marieke Op de Beeck, Floris L van Delft and Annemieke Madder (UGent)
2'-O-(3-(Furan-2-yl)propyl)adenosine was synthesized and evaluated for interstrand crosslink (ICL) formation in DNA duplexes. In situ oxidation of the furan moiety with NIS showed rapid crosslink formation to dA and dC, while dT and dG were inactive.
(2011) CHEMICAL COMMUNICATIONS. 47(10). p.2796-2798
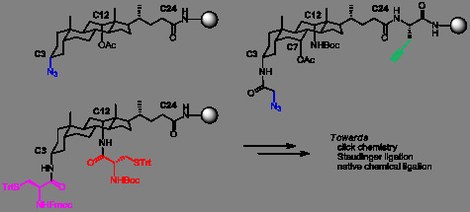
32. Shortcut access to peptidosteroid conjugates : building blocks for solid-phase bile acid scaffold decoration by convergent ligation
Dieter Verzele (UGent), Sara Figaroli and Annemieke Madder (UGent)
We present three versatile solid-supported scaffold building blocks based on the (deoxy) cholic acid framework and decorated with handles for further derivatization by modern ligation techniques such as click chemistry, Staudinger ligation or native chemical ligation. Straightforward procedures are presented for the synthesis and analysis of the steroid constructs. These building blocks offer a new, facile and shorter access route to bile acid-peptide conjugates on solid-phase with emphasis on heterodipodal conjugates with defined spatial arrangements. As such, we provide versatile new synthons to the toolbox for bile acid decoration.
(2011) MOLECULES. 16(12). p.10168-10186
2010
31. Design and automated generation of artificial estrogen receptor as potential endocrine disruptor chemical binders
We here report on our results concerning the systematic design and synthesis of a new type of artificial estrogen receptor candidates. Our interest herein originates from the known estrogenic activity of endocrine disrupting chemicals, environmental pollutants that have attracted increased attention due to their interference with the endocrine and reproductive system of wildlife and humans. An automated protocol for the generation of libraries of potential artificial receptors is described. The resulting structures could find application in novel SPE cartridges for EDC-preconcentration.
Sara Figaroli and Annemieke Madder (UGent)
(2010) TETRAHEDRON. 66(34). p.6912-6918
30. Surprising duplex stabilisation upon mismatch introduction within triply modified duplexes
Mieke Catry (UGent) , Vicky Gheerardijn (UGent) and Annemieke Madder (UGent)
Three different modified phosphoramidite nucleoside building blocks equipped with additional protected imidazole, masked alcohol and masked carboxylate functionality are synthesized and incorporated into oligonucleotides. Based on the serine-protease active site model, doubly and triply modified duplexes are created and tested for stability. Analysis of different spatial distributions of the extra functionalities shows that careful positioning can even overcome duplex destabilisation caused by the introduction of mismatches.
(2010) BIOORGANIC CHEMISTRY. 38(1-3). p.92-97
29. Peptide scalpels for site-specific dissection of the DNA-protein interface
Dieter Verzele (UGent) , Lieselot Carrette (UGent) and Annemieke Madder (UGent)
Current review highlights emerging strategies for the design of DNA-binding peptides and crosslinking techniques capable of interference at the DNA–protein interface with emphasis on bottom-up organic synthesis. Despite the obvious fundamental character, these inquiries could ultimately result in interesting biomedical applications as designed genome interfering agents or diagnostics. Recent awareness of the potential of these larger peptidomimetic constructs has risen within the medicinal society.
(2010) DRUG DISCOVERY TODAY. TECHNOLOGIES. 7(2). p.e115-e123
28. NF-31 color test uncovers 'hidden' alcohol functionalities in PEG-based resins for solid phase peptide synthesis
Lieselot Carrette (UGent) , Dieter Verzele (UGent) and Annemieke Madder (UGent)
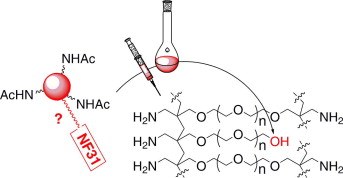
(2010) TETRAHEDRON LETTERS. 51(16). p.2106-2108
2009
27. Furan-modified oligonucleotides for fast, high-yielding and site-selective DNA inter-strand cross-linking with non-modified complements
Kristof Stevens (UGent) and Annemieke Madder (UGent)
Among the various types of DNA damage, inter-strand cross-links (ICL) represent one of the most cytotoxic lesions. Processes such as transcription and replication can be fully blocked by ICLs, as shown by the mechanism of action of some anticancer drugs. However, repair of ICLs can be a possible cause of resistance. To study the mechanisms of cross-link repair stable, site-specifically cross-linked duplexes are needed. We here report on the synthesis of site-specifically cross-linked DNA using an acyclic furan containing nucleoside. Selective in situ oxidation of the incorporated furan moiety generates a highly reactive oxo-enal that instantly reacts with the complementary base in a non-modified strand, yielding one specific stable cross-linked duplex species. Varying sequence context showed that a strong selectivity for cross-linking to either complementary A or complementary C is operating, without formation of cross-links to neighboring or distant bases. Reaction times are very short and high isolated yields are obtained using only one equivalent of modified strand. The formed covalent link is stable and the isolated cross-linked duplexes can be stored for several months without degradation. Structural characterization of the obtained ICL was possible by comparison to the natural mutagenic adducts of cis-2-butene-1,4-dial, a metabolite of furan primarily responsible for furan carcinogenicity.
(2009) NUCLEIC ACIDS RESEARCH. 37(5). p.1555-1565
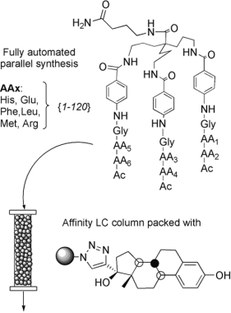
26. Towards a New SPE Material for EDCs: Fully Automated Synthesis of a Library of Tripodal Receptors Followed by Fast Screening by Affinity LC
Steven Van der Plas (UGent) , Els Van Hoeck (UGent) , Frederic Lynen (UGent) , Patrick Sandra (UGent) and Annemieke Madder (UGent)
A series of human estrogen receptor (hER) mimics were synthesised. On the basis of the knowledge on the structure of the hormone binding domain of the hER, three different peptide chains were constructed onto a tripodal scaffold. By using a fully automated solid-phase synthesis protocol, 120 members with known identity were synthesised in only a week. Affinity towards estrogenic compounds was checked by affinity LC. For this purpose, ethinylestradiol was "clicked" onto a modified-silica phase, and the obtained material was packed into an HPLC column. The results stemming from the affinity LC experiments were confirmed by clicking one library member to silica and by using this solid phase to extract different endocrine-disrupting chemicals (EDCs) from aqueous media.
(2009) EUROPEAN JOURNAL OF ORGANIC CHEMISTRY. 11(Sp. Iss. SI). p.1796-1805
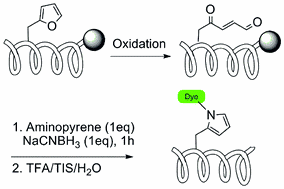
25. From DNA cross-linking to peptide labeling: on the versatility of the furan-oxidation-conjugation strategy
Annelies Deceuninck (UGent) and Annemieke Madder (UGent)
Based on the incorporation of commercially available N-Fmoc-furylalanine, a new method for peptide labeling is proposed, relying on the selective oxidative transformation of the furan moiety into a reactive aldehyde and subsequent reductive amination.
(2009) CHEMICAL COMMUNICATIONS. 3. p.340-342
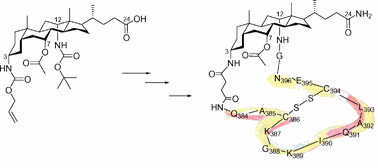
24. Towards the conformational mimicry of the measles virus HNE loop: design, synthesis and biological evaluation of a cyclic bile acid-peptide conjugate
Catherine Bode, Tom Bechet, Emmanuel Prodhomme, Katelijne Gheysen, Pieter Gregoir (UGent) , José Martins (UGent) , Claude Muller and Annemieke Madder (UGent)
The current article reports the design, synthesis and biochemical evaluation of a cyclic bile acid-peptide conjugate as a mimic of the loop-like structure of the measles virus haemagglutinin noose epitope (HNE). This macrocyclic structure was assembled by solid phase synthesis. Scaffold-peptide ring closure was achieved via the introduction of a succinate linker. After disulfide bridge formation with iodine, the desired 14 amino acid cyclic conjugate was obtained with overall yields between 15 and 35%. NMR analysis supports the presence of a helical conformation in the Q384-G388 pentapeptide portion, in agreement with the organisation of this chain in the native protein. The compound was found to have increased biostability compared to stabilised linear peptides, displayed good binding towards monoclonal antibodies known to bind to HNE and thus has potential in an alternative peptide-based measles vaccine.
(2009) ORGANIC & BIOMOLECULAR CHEMISTRY. 7(17). p.3391-3399
23. Proteome-wide substrate analysis indicates substrate exclusion as a mechanism to generate caspase-7 versus caspase-3 specificity
Dieter Demon (UGent) , Petra Van Damme (UGent) , Tom Vanden Berghe (UGent) , Annelies Deceuninck (UGent) , Joost Van Durme (UGent) , Jelle Verspurten (UGent) , Kenny Helsens (UGent) , Francis Impens (UGent) , Magdalena Wejda (UGent) , Joost Schymkowitz, et al.
Caspase-3 and -7 are considered functionally redundant proteases with similar proteolytic specificities. We performed a proteome-wide screen on a mouse macrophage lysate using the N-terminal combined fractional diagonal chromatography technology and identified 46 shared, three caspase-3-specific, and six caspase-7-specific cleavage sites. Further analysis of these cleavage sites and substitution mutation experiments revealed that for certain cleavage sites a lysine at the P5 position contributes to the discrimination between caspase-7 and -3 specificity. One of the caspase-7-specific substrates, the 40 S ribosomal protein S18, was studied in detail. The RPS18-derived P6-P5' undecapeptide retained complete specificity for caspase-7. The corresponding P6-P1 hexapeptide still displayed caspase-7 preference but lost strict specificity, suggesting that P' residues are additionally required for caspase-7-specific cleavage. Analysis of truncated peptide mutants revealed that in the case of RPS18 the P4-P1 residues constitute the core cleavage site but that P6, P5, P2', and P3' residues critically contribute to caspase-7 specificity. Interestingly, specific cleavage by caspase-7 relies on excluding recognition by caspase-3 and not on increasing binding for caspase-7.
(2009) MOLECULAR & CELLULAR PROTEOMICS. 8(12). p.2700-2714
2008
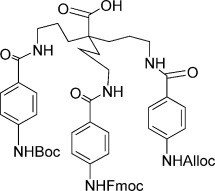
22. Synthesis of a tripodal scaffold for solid phase synthesis of artificial receptors
Steven Van der Plas (UGent) , An Gea, Sara Figaroli, Pierre De Clercq (UGent) and Annemieke Madder (UGent)
A convergent synthesis for the preparation of a new orthogonally protected tripodal scaffold has been developed. The scaffold was successfully coupled to a Tentagel solid support and further derivatised at the three attachment points demonstrating its possible use for combinatorial chemistry.
(2008) EUROPEAN JOURNAL OF ORGANIC CHEMISTRY. p.1582-1588
2007
21. LC-(TIC/EIC)-MS as tool in the analysis of diastereomeric 3,12-aza-analogues of deoxycholic acid
Dieter Verzele (UGent) , Jan Goeman (UGent) and Annemieke Madder (UGent)
The non-conventional Total Ion Current (TIC) detection and corresponding Extracted Ion Current (EIC) chromatogram in LC-MS measurements, have been used for quantitative analysis of reactions involving UV-inactive aza-analogues of deoxycholic acid. LC-(TIC/EIC)-MS experiments allowed for the accurate determination of diastereomeric and overall purity during the development of a new synthetic route for an orthogonally protected dipodal steroid scaffold with defined stereochemistry. Moreover, this technique facilitated adequate monitoring of further scaffold derivatization on solid support. The described methodology offers an attractive solution in cases where conventional HPLC analysis with UV detection fails.
20. Synthesis and incorporation of a simple acyclic furan containing phosphoramidite
Kristof Stevens (UGent) and Annemieke Madder (UGent)
A novel furan containing phosphoramidite was synthesized and incorporated into model oligonucleotides. This glycol nucleic acid based building block contains a furan unit substituting the natural base, and can be used for post synthetic oligonucleotide modifications by orthogonal chemistries such as Schiff base formation after in situ oxidation or Diels-Alder cycloadditions.
(2007) NUCLEOSIDES NUCLEOTIDES & NUCLEIC ACIDS. 26(10-12). p.1359-1362
19. Validation of a solid-phase-bound steroid scaffold for the synthesis of novel cyclic peptidosteroids
Catherine A. Bodé, Claude P. MULLER and Annemieke Madder (UGent)
The current article reports on the synthesis of a new type of cyclic peptidosteroid, in which a bile-acid-based scaffoldwas used for the conformational restriction of a loop-like peptide. Convergent coupling of two tetrapeptides to the non-peptidicsteroidal entity was carried out once in the classical C-to-N and once in the non-classical N-to-C direction. Peptide backbonecyclisation was then carried out, giving rise to a ring size equivalent to approximately 12 amino acids. This type of construct will be used in the development of a peptide vaccine against measles.
(2007) JOURNAL OF PEPTIDE SCIENCE. 13(11). p.702-708
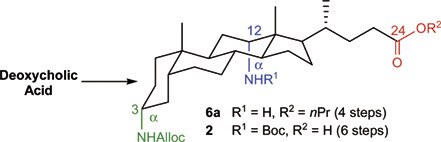
18. Short synthesis of orthogonally protected 3 alpha,12 alpha-diamino-5 beta-cholan-24-oic acid, a dipodal steroid scaffold for combinatorial chemistry
Dieter Verzele (UGent) and Annemieke Madder (UGent)
A short, practical, multigram-scale synthesis of C3α-NHAlloc, C12α-NHBoc-diamino-5β-cholan-24-oic acid 2 was developed, applying a new, straightforward synthetic strategy. Key features are the conservation of the carboxyl moiety at C24 during oxime reduction, the late differentiation between the C3 and C12 amino groups and the gradual separation of diastereomers during the synthesis. This orthogonally protected diamino steroid derivative can be used as starting point for the generation of steroid based dipodal peptide and non-peptide combinatorial libraries
(2007) EUROPEAN JOURNAL OF ORGANIC CHEMISTRY. p.1793-1797
17. Fast and easy detection of aromatic amines on solid support
Steven Van der Plas (UGent) , Pierre De Clercq (UGent) and Annemieke Madder (UGent)
The use of p-nitrophenyl ester 1 has been shown to offer a reliable method for the detection of free aromatic amines. As little as 3.4 μmol g−1 of free aniline amino groups can be detected. The method has shown to be more sensitive for the detection of sterically hindered aromatic amines than the existing alternative based on reaction with chloranil.
(2007) TETRAHEDRON LETTERS. 48(14). p.2587-2589
16. Synthesis of functionalised nucleosides for incorporation into nucleic acid-based serine protease mimics
Mieke Catry (UGent) and Annemieke Madder (UGent)
The synthesis of nucleosides modified with an extra imidazole, carboxyl and hydroxyl group is described. These nucleosides can be incorporated into an oligonucleotide duplex, thus generating a novel type of serine protease mimic.
(2007) MOLECULES. 12(1). p.114-129
2006
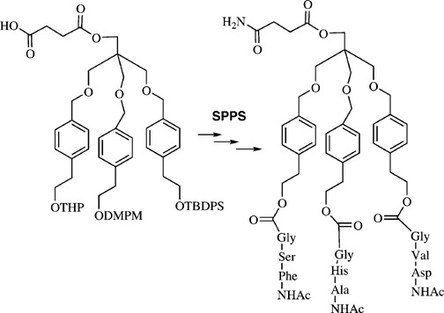
15. Solid-supported synthesis of highly functionalized tripodal peptides with flexible but preorganized geometry: towards potential serine protease mimics
An Gea, Nadia Farcy, Núria Roqué i Rossell, José Martins (UGent) , Pierre De Clercq (UGent) and Annemieke Madder (UGent)
Tripodal scaffold 1 has been used in the synthesis of a representative member of a library of serine protease mimics, possessing three independent functionalized peptide chains on a central core. Each peptide chain contains one residue of the classical catalytic triad (serine, histidine and aspartate) found in the active site of the serine protease α-chymotrypsin. A particular feature of the novel tripodal design is its essentially flexible yet preorganized structure as deduced from molecular modeling studies. The choice of suitable scaffold and side-chain protecting groups was intensively studied and optimized to ensure complete orthogonality. Inclusion of a photolabile linker enabled the release of intermediates and final structures from the solid-support polymer allowing positive evaluation of the present strategy for the synthesis of future tripodal libraries of serine protease mimics
(2006) EUROPEAN JOURNAL OF ORGANIC CHEMISTRY. p.4135-4146
2005
14. Fine-tuning furan toxicity: fast and quantitative DNA interchain crosslink formation upon selective oxidation of a furan containing oligonucleotide
S. Halila, T. Velasco, Pierre De Clercq (UGent) and Annemieke Madder (UGent)
Oligonucleotides containing a furan modified internal nucleoside have been synthesized; selective in situ oxidation of the furan moiety to a reactive enal species in the presence of a complementary DNA strand gives rise to fast and efficient formation of an interstrand cross-link.
(2005) CHEMICAL COMMUNICATIONS. p.936-938
2003
13. Stabilisation of RNA bulges by oligonucleotide complements containing an adenosine analogue
Annemieke Madder (UGent), R. Ehrl and R. Strömberg
Incorporation of 2′-deoxy-2′-β-(1-naphthylmethyl)tubercidin into an oligodeoxyribonucleotide mostly has little or a slightly negative effect on the Tm values of complexes with DNA complements. With the same naphthylmethyl-substituted nucleoside at the 3′-end of a 2′-O-methyloligoribonucleotide, however, a stabilisation of 1–2 °C in the corresponding complexes with both DNA and RNA is observed. When the target sequence is an RNA fragment forming a two- or three-nucleotide bulge, complexes with (naphthylmethyl)tubercidin-modified oligodeoxyribonucleotides, as well as with the corresponding 2′-O-methyloligoribonucleotides, give stabilisations of 1–2 °C for the three-nucleotide bulge and of almost 4 °C for the two-nucleotide bulge. This stabilisation is specific to RNA, since the corresponding complexes with the DNA fragments do not display this effect. Thus, the (naphthylmethyl)tubercidin-containing oligonucleotides are the first reported oligonucleotide modifications that specifically stabilise bulged RNA.
(2003) CHEMBIOCHEM. 4(11). p.1194-1200
12. Stabilization of RNA bulges by oligonucleotides containing 2 '-naphthylmethyl-2 '-deoxytubercidine
Annemieke Madder (UGent), R. Ehrl and R. Strömberg
A novel nucleoside analogue, 2'-naphthylmethyl-2'-deoxytubercidine, is synthesized and incorporated in oligonucleotides that stabilize bulges in partially complementary RNA.
(2003) NUCLEOSIDES NUCLEOTIDES & NUCLEIC ACIDS. 22(5-8). p.1289-1291
2002
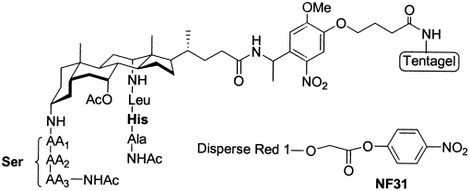
11. Evaluation of a two-stage screening procedure in the combinatorial search for serine protease-like activity.
Annemieke Madder (UGent) , L LI, Hilde De Muynck, Nadia Farcy, Dirk Van Haver (UGent) , Franky Fant, G Vanhoenacker, Patrick Sandra (UGent) , AP DAVIS and Pierre De Clercq (UGent)
A series of peptidosteroid derivatives containing two independent peptide chains in which Ser and His are incorporated were synthesized by solid-phase peptide synthesis. The activity of the different compounds in the hydrolysis of the activated substrate NF31 was assessed in a stepwise fashion. First, the different resin-bound derivatives 6a-1 and 6x-z were individually assayed for serine esterification in the absence of water. The use of a colored substrate allowed for a visual identification of the most active compounds. Through the inclusion of control substances, the involvement of histidine in the mechanism for serine acylation was shown. Second, the hydrolysis and methanolysis of the different acylated derivatives 8a-1 and 8x were evaluated using UV spectroscopy, again indicating the involvement of histidine. The feasibility of applying the above procedures in a combinatorial context was proven via the screening of artificial libraries, created by mixing the different resin-bound peptidosteroid compounds. In this respect, the use of a photocleavable linker allowed for the unambiguous structural characterization of the selected members via application of single-bead electrospray tandem mass spectrometry.
(2002) JOURNAL OF COMBINATORIAL CHEMISTRY. 4(6). p.552-562
10. Peptide sequence determination of peptidosteroids on a single bead by electrospray ionization-mass spectroscopy.
G. Van Hoenacker, L. Liu, Frederic Lynen (UGent) , Annemieke Madder (UGent) , Pierre De Clercq (UGent) and Patrick Sandra (UGent)
An electrospray ionization mass spectroscopy (ESI-MSn) method has been developed to simultaneously determine the sequence of two strands of tripeptides attached to a steroid-like scaffold. The peptidosteroid compounds were synthesized by solid-phase chemistry on TentaGel beads. The sequence of both peptide strands of a compound originating from a single bead could be determined. High-performance liquid chromatography hyphenated to MSn was also applied to a mixture of two isomeric peptidosteroids for sequence determination of their peptide chains.
(2002) JOURNAL OF SEPARATION SCIENCE. 25(10-11). p.671-676
2001
9. A pentaerythritol-based molecular scaffold for solid-phase combinatorial chemistry.
Nadia Farcy, Hilde De Muynck, Annemieke Madder (UGent) , N HOSTEN and Pierre De Clercq (UGent)
A convergent synthesis has been developed for the preparation of solid-phase bound construct 1, consisting of an orthogonally protected trifunctional core structure that is attached to TentaGel via a photocleavable linker.
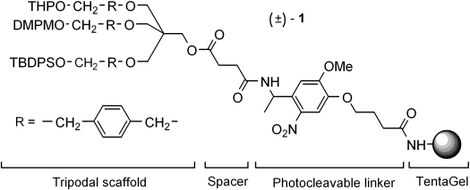
(2001) ORGANIC LETTERS. 3(26). p.4299-4301
2000
8. Rationally designed bicyclic lactams control different turn motifs and folding patterns in hexapeptide mimics.
L. Belvisi, C. Gennari, Annemieke Madder (UGent) , A. Mielgo, D. Potenza and C. Scolastico
Conformational analysis of N-acetylated hexapeptide mimics incorporating a bicyclic lactam (1-4) was carried out by a combination of 1H-NMR spectroscopy, IR spectroscopy, and computer modeling. The nature of the bicyclic lactam determines the turn motifs and the folding patterns of these constrained peptides. The (5,6)-bicyclic lactam derivatives 1 and 2, characterized by a type-II' β-turn (CO3···H6-N), are very compact intramolecularly H-bonded structures. The (5,7)-bicyclic lactam derivative 3, characterized by an inverse γ-turn (CO4···H6-N), is a quite flexible “tweezer-like” structure.
(2000) EUROPEAN JOURNAL OF ORGANIC CHEMISTRY. 5. p.695-699
7. Application of combinatorial procedures in the search for serine-protease-like activity with focus on the acyl transfer step.
Hilde De Muynck, Annemieke Madder (UGent) , Nadia Farcy, Pierre De Clercq (UGent) , MN. Perez-Payan, LM. Ohberg and AP Davis
Recursive deconvolution of a 729-membered peptide library has identified three active sequences, in which both Ser and His are present in one of the two tripeptidic chains generated on a steroidal scaffold (see structural formula), for the cleavage of an activated p-nitrophenyl ester. This combinatorial approach aims at searching for serine-protease-like activity.
(2000) ANGEWANDTE CHEMIE-INTERNATIONAL EDITION. 39(1). p.145-148
1999
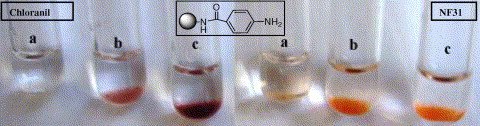
6. Novel sensitive colorimetric assay for visual detection of solid-phase bound amines
Annemieke Madder (UGent) , Nadia Farcy, NGC Hosten, Hilde De Muynck, Pierre De Clercq (UGent) , J Barry and APA Davis
A new simple and efficient method for the detection of incomplete coupling reactions during solid-phase peptide synthesis is described. Using p-nitrophenyl ester 1 (NF31), free amino groups can be visually detected on the resin by direct coloring of the beads. A specific feature of the assay resides in the possibility of detection of sterically hindered primary amines.
(1999) EUROPEAN JOURNAL OF ORGANIC CHEMISTRY. 11. p.2787-2791
1998
5. Stepwise approach toward first generation nonenzymatic hydrolases
Annemieke Madder (UGent) , Pierre De Clercq (UGent) and Jean-Paul Declercq
The synthesis and reactivity study of a first generation serine protease mimic is described. Central in the design stands the possibility of stabilization of the transition state by an amino triol such as 8t. En route to 8t, a series of amino alcohols (4-8) was obtained, the reactivity of which was studied toward esterification by acetylimidazole (AcIm) and by p-nitro-2,2,2-trifluoroacetanilide (PNTFA). Interesting reactivity differences were observed between the cis-and the trans-series, especially between 7c and 7t (AcIm), and between 8c and 8t (PNTFA). In both cases the results are explained by invoking extra stabilization of the tetrahedral oxyanion.
(1998) JOURNAL OF ORGANIC CHEMISTRY. 63(8). p.2548-2559
1997
4. Mechanism of esterification of 1,3-dimethylamino alcohols by N-acetylimidazole in acetonitrile and the influence of alkyl and geminal dialkyl substitution upon the rate.
Annemieke Madder (UGent) , S Sebastian, Dirk Van Haver (UGent) , Pierre De Clercq (UGent) and H Maskill
3-(Dimethylamino)propan-1-ol and seven derivatives with alkyl substituents at the 2-position have been prepared by conventional methods, and second-order rate constants for their esterification by N-acetylimidazole in acetonitrile have been measured under pseudo-first-order conditions both by H-1 NMR spectroscopy at a single temperature (23 degrees C) and by a UV spectroscopic method over the temperature range 25-65 degrees C. Evidence is presented that the intermolecular esterifications proceed via an initial rate-determining intramolecular general base catalysed formation of a cyclic tetrahedral intermediate. Effective molarities compared with the third-order reactions of simpler alcohols with acetylimidazole catalysed by triethylamine are estimated to be 13-14 mol dm(-3), but alkyl substitution at the 2-position of the amino alcohol has only a modest effect upon reaction rates. All reactions have substantial negative entropies of activation and only modest enthalpies of activation as expected for concerted bimolecular reactions with highly ordered transition structures. Three structurally related carbocyclic amino alcohols constitute a short isokinetic series with the isokinetic temperature very close to the experimental range. Along this series, decreasing enthalpies of activation are almost exactly balanced in their contributions to the overall free energy of activation near room temperature by increasingly negative entropies of activation.
(1997) JOURNAL OF THE CHEMICAL SOCIETY-PERKIN TRANSACTIONS 2. 12. p.2787-2793
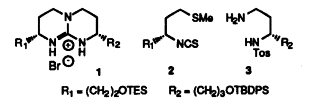
3. The synthesis of di(hydroxyalkyl) substituted bicyclic guanidines.
Annemieke Madder (UGent) , I Munster, U Rolle and Pierre De Clercq (UGent)
The synthesis of the enantiomerically pure bicyclic guanidines 3a and 3b is described. The convergent strategy is based on methodology developed by Schmidtchen. The therefore required amine components 6 and 9 are obtained from methionine and L-glutamic acid, respectively.
(1997) BULLETIN DES SOCIETES CHIMIQUES BELGES. 106(9-10). p.613-621
2. Structure-reactivity relationships in the rate of esterification by acetylimidazole: The influence of the second hydroxy group and of the length of the N-omega-hydroxy-n-alkyl chain in 3-(N-methyl, N-omega-hydroxy-n-alkyl)amino-2-tert-butylpropan-1-ols.
Annemieke Madder (UGent) , Pierre De Clercq (UGent) and H Maskill
Enforced intramolecular hydrogen bonding facilitates intramolecular general base catalysis in the acetylation of a family of α,ω-amino alcohols by acetylimidazole, and the site of acetylation when there are two hydroxy groups is determined by the relative ease of intramolecular hydrogen bonding rather than by intermolecular steric effects.
(1997) JOURNAL OF THE CHEMICAL SOCIETY-PERKIN TRANSACTIONS 2. 5. p.851-852
1995
1. Synthesis of a chiral di(hydroxyalkyl) substituted bicyclic guanidine
I Munster, U Rolle, Annemieke Madder (UGent) and Pierre De Clercq (UGent)
The chiral regioselectively functionalized bicyclic guanidinium salt 1 is obtained from isocynate 2 (from L-methionine) and amine 3 (from L-glutamic acid) according to Schmidtchen's methodology.
(1995) TETRAHEDRON-ASYMMETRY. 6(11). p.2673-2674